- The U.S. FDA has accelerated approval for Kebilidi (eladocagene exuparvovec-tneq), a one-time AAV2 gene therapy for AADC deficiency.
- It is the first FDA-approved gene therapy administered directly into the brain (intraputaminal infusion).
- Indicated for adult and pediatric patients with AADC deficiency.
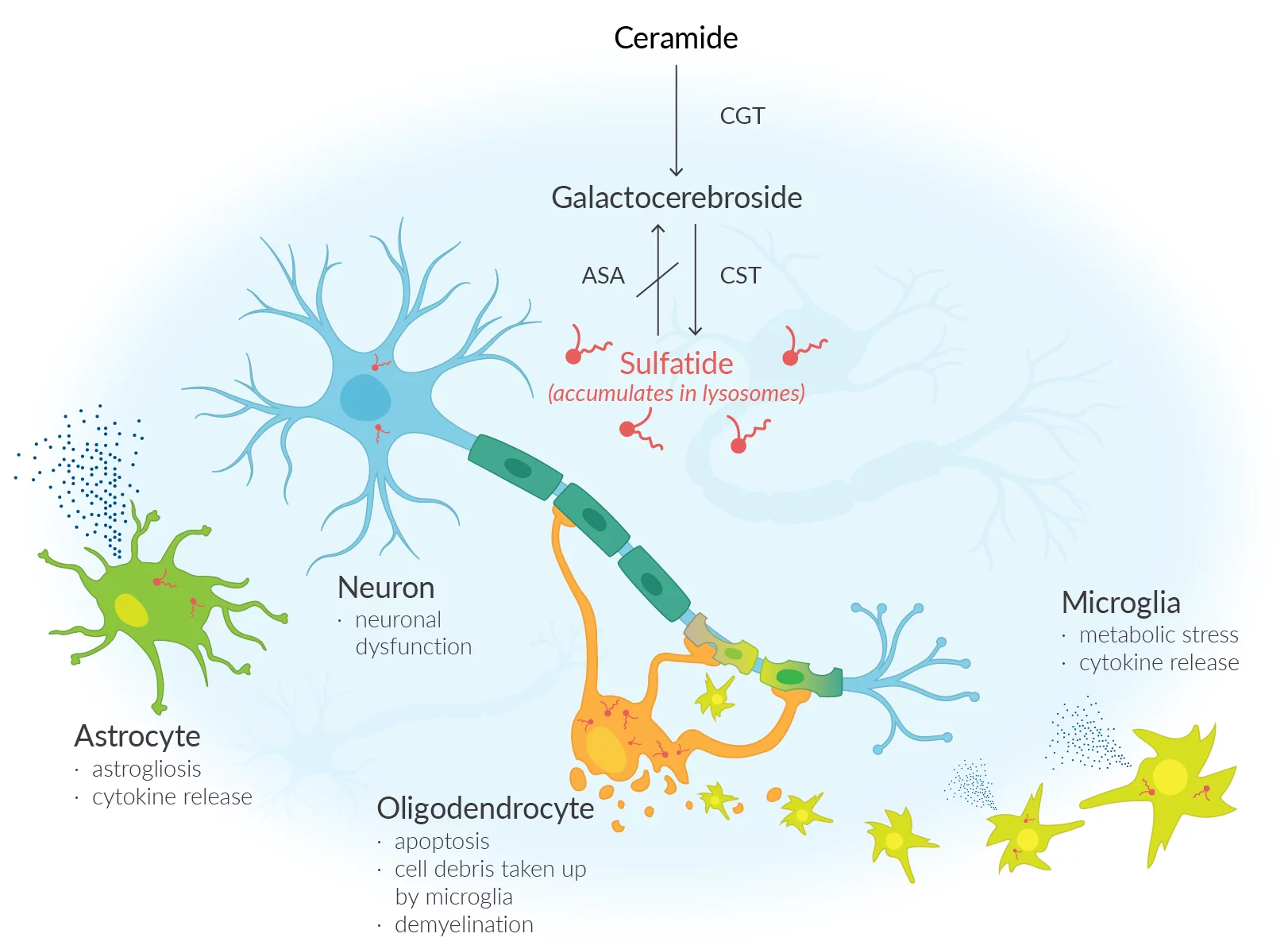
The U.S. Food and Drug Administration has granted accelerated approval to Kebilidi (eladocagene exuparvovec-tneq), marking the first U.S. gene therapy delivered via direct intracerebral (intraputaminal) administration. This milestone expands treatment options for aromatic L-amino acid decarboxylase (AADC) deficiency, a rare, debilitating neurogenetic disorder with historically limited disease-modifying therapies.